Hospitals that dispense injection pens to administer insulin to inpatients face unusual labeling challenges. A pharmacy professional used a solution that includes BarTender® label software to devise an innovative labeling system that improves patient safety.
The challenge:
Safe insulin pen usage in hospitals

Although the pens were designed for patient self-injection and at-home use, they also became the method of choice for hospitals to administer insulin at bedside, creating a unique set of problems.
Cross-contamination
When a vial and syringe is used to administer insulin, the same vial could be used with multiple patients. However, the cartridge of an insulin pen is not the same as a vial. “Even though the disposable needle is replaced after each injection, intact human cells can travel though the needle and into the cartridge during injection,” said Chuck DiTrapano, RPh, the operations manager at the pharmacy of a major US health facility. Some hospitals did not recognize this difference and initially failed to limit a cartridge’s use to one patient, leading to cross-contamination. In March 2009, the US Food and Drug Administration responded to the problem with an alert that multiple-patient use of an insulin pen can transmit disease. The FDA cited incidents at two hospitals where shared use of insulin pens potentially exposed an estimated 2,000 patients to Hepatitis C and HIV, even though needles were changed after each injection. Even when a hospital does limit cartridge use to one patient, cross-contamination can occur due to simple human error, such as when insulin pens are mixed up from patient to patient at bedside.
Expiration dates

All medications have an expiration date and require proper handling to ensure efficacy. In the case of insulin pens, they must be transported from the pharmaceutical manufacturer and stored in the pharmacy under refrigeration. “Once a pen is removed from refrigeration and dispensed, its window of use changes,” said DiTrapano. Unrefrigerated insulin expires after anywhere from 7 to 42 days (7, 14, 28, 30 or 42) when stored at room temperature, depending on the product, product volume and brand, so the pharmacist must calculate the new expiration date and mark it on the pen when it is dispensed. To maintain patient safety, it is imperative that a drug be used within its particular window, but accurately managing the wide variety of use and disposal dates can be complicated for a hospital staff.
Labeling considerations
Safe use of an insulin pen hinges on single- patient use, clear patient identification, and a room temperature expiration date on the device.
Identification best practices
The Institute for Safe Medical Practices (ISMP) has defined strict best practices for labeling. To protect patient safety, a label must feature two forms of patient identification, such as name and medical record number, proper storage condition information, proper drug ID information and proper expiration date.
The ISMP also advises a font strategy called Tall Man lettering, which uniquely combines upper- and lower-case characters to enable easy differentiation and identification of similarly named drugs, thereby helping health care professionals avoid potentially dangerous dosing mix-ups.
Impediments to labeling
Unfortunately, insulin pens are not easy to label. “There are very expensive dispensing software applications that can calculate expiration and dose and print that information on a label,” DiTrapano said, “but their label placement is inadequate—the patient label can either be placed on the shaft of the pen, obscuring the product info, or on the pen’s cap, and a nurse juggling two pens can easily place the labeled cap on the wrong pen.”
Also, the pen’s small diameter makes it difficult to legibly print all the required information.
The solution: RxTOOLKIT® and BarTender® label software
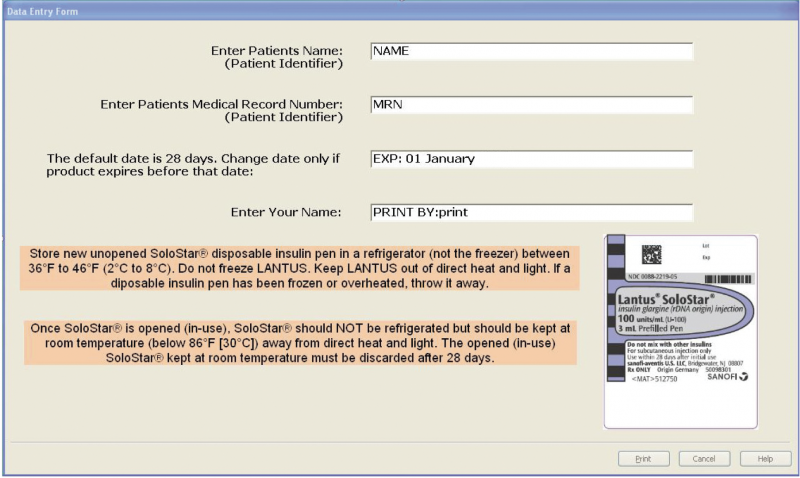
DiTrapano first started using BarTender in 2004. “Our hospital mandated new procedures to ensure patient safety, including a protocol for scanning drug labels and patient wristbands when a drug was administered bedside,” he said. “I went looking for a pharmaceutical barcode labeling system to help meet the mandate, but there was nothing commercially available at the time that filled our needs.”
While some pharmacy information systems could generate labels, none could provide the customization and interconnectivity between systems that a regional health facility’s pharmacy required.
And none could generate Tall Man text. “ISMP gives us guidelines and not regulations, but we’re meticulous about best practices. Patient safety is our primary concern, and not having Tall Man text is a deal breaker,” DiTrapano said.
In addition to his role as a pharmacy professional, DiTrapano is also president of HMMRx, whose mission is to improve medication safety. To address the real-world patient safety challenges that DiTrapano was encountering, his HMMRx business partner and CIO, John Neville, created a new pharmacy labeling safety management solution, RxTOOLKIT.®
BarTender® label software from Seagull Scientific is an integral part of RxTOOLKIT’s labeling solution. In addition to label design and printing, the system uses VB Scripting in BarTender to automatically calculate doses, capture patient identification information, and tie into national pharmaceutical databases for each drug dispensed. The combined solution is designed to meet the exacting dispensing requirements of applications ranging frozm NICU and pediatrics to the precise IV infusion calculations and dosage tapering of immunoglobulin therapy.
For products that need to be blended or reconstituted in the pharmacy, RxTOOLKIT retrieves product information from national databases to determine how much of each active ingredient, excipient or diluent needs to be included, based on the drug and specified dosage for the individual patient.
Labeling insulin pens
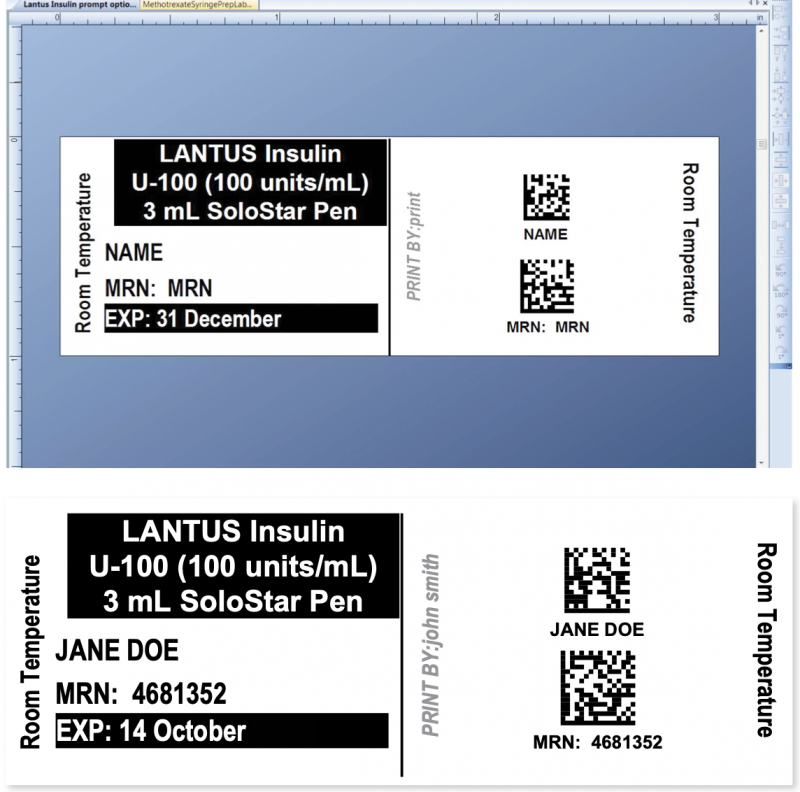
The hospital use of insulin pens was particularly suited to an RxTOOLKIT and BarTender solution.
Using the combined solution to create a dispensing label, the pharmacist can either scan the code on the insulin pen’s manufacturer label or type the word “Insulin” into the provided field. In the latter case, a drop-down menu of insulin brand names appears. Once a product is selected, the label displays in the formatting window.
The expiration information is automatically filled in based on room temperature expiration dates for the brand of insulin pen chosen.
The pharmacist then scans or types the patient MRN or name. The template automatically applies ISMP best practices, including the use of all upper case letters for the patient, all lower case letters for the dispensing pharmacist, and two forms of patient ID.
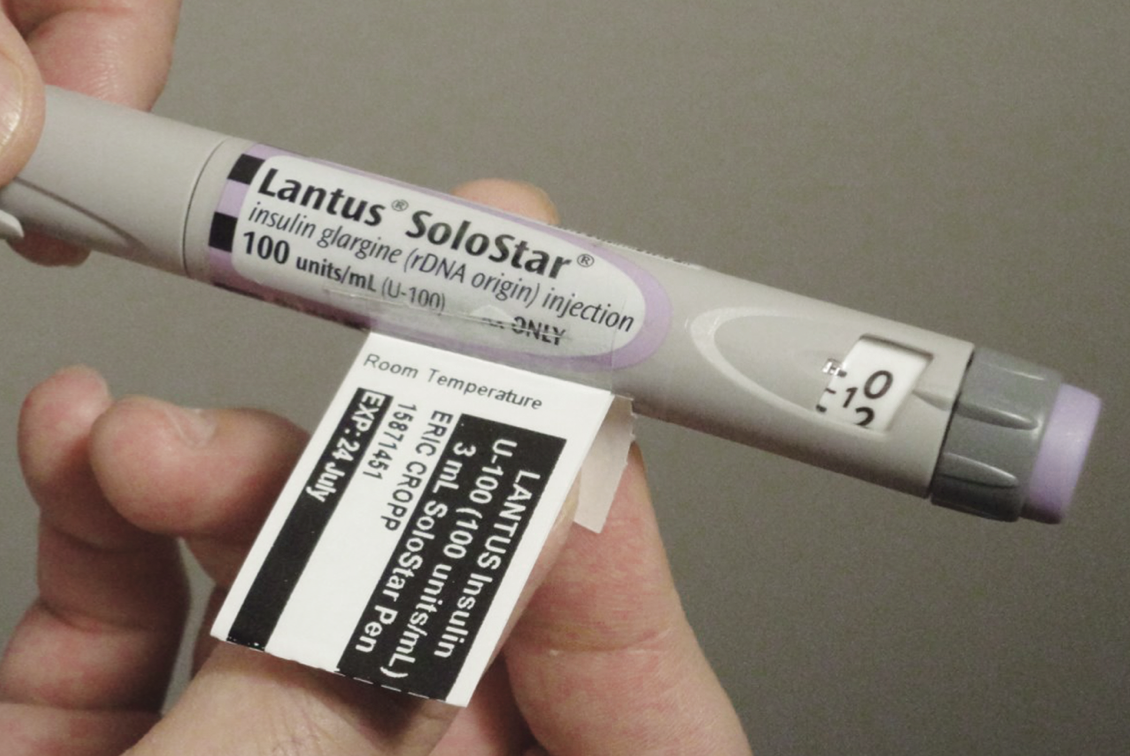
Once the label is printed, it’s affixed to the pen via a new and innovative method of attaching pharmacy labels, devised by DiTrapano specifically for insulin pen applications. Working with TSI Associates, he devised the Clear Connect Label, TM a transparent adhesive sleeve that fastens the patient label to the insulin pen as a tag. All the patient, dosing and expiration information required to safely dispense and use the insulin pen can be displayed without obscuring the manufacturer label information.
The benefits
RxTOOLKIT is now deployed in hospital pharmacies throughout the United States. RxTOOLKIT insulin pen labels connect key staff members across health care disciplines, using barcodes generated with BarTender labeling software to make accurate, critical data immediately and easily accessible. Nurses spend less time looking for drug dosage and resource information, and more time caring for their patients, while reliable patient identification lessens the risk for patient mix-ups. And because BarTender automates crucial calculations, the potential for error is reduced, which results in improved confidence and efficiency among the clinical staff. RxTOOLKIT and BarTender are integral to communication, providing enhanced patient safety, streamlined processes and reduced costs.
Article Credit – https://www.seagullscientific.com/media/2157/bartender-case-study-insulin-en.pdf